Biopharma processing
Insulin
Overview
Overview
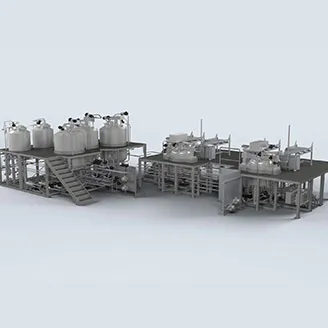
Insulin pharmaceutical manufacturing involves a complex, highly regulated process. It begins with the cultivation of genetically modified bacteria or yeast, such as Escherichia coli or Saccharomyces cerevisiae, which produce human insulin analogs. Fermentation tanks are used to grow these microorganisms and generate insulin.
After fermentation, the cells are harvested, and insulin is extracted and purified through multiple filtration and chromatography steps. This yields a highly purified insulin solution. Further processing may include formulation with stabilizers and preservatives. The entire plant must be fully cleanable and sterilizable.
Control system is the key to success for this application, considering the batch can last up to several days: chromatographic column and other process packages may be necessary.
Olsa has the capability, process-knowledge, and expertise to offer tailored solutions for insulin production, encompassing various preparation vessel configurations and a wide range of automation options. Thanks to the synergy with MGA (Masco Group Automation), Olsa can provide a high-level automation package (for example SCADA, DCS) able to handle complex receipts to guarantee process control and reporting according with highest pharmaceutical and biopharmaceutical standards.
Other Biopharma processing applications