Biopharma processing
Opthalmic products
Overview
Overview
Ophthalmic solutions, such as eye drops or artificial tears, and ophthalmic gel or ointments are manufactured using a combination of pharmaceutical and sterile manufacturing processes to ensure they are safe and effective for use in the eyes.
The specific manufacturing steps can vary depending on the type of product and the active ingredients involved, but a general overview of the typical manufacturing process involves, after formulation development and raw materials preparation (APIs, excipients, purified water), two main steps: sterilization or aseptic processing, critical to prevent microbial contamination, using sterile manufacturing environments, equipment, and techniques; mixing and homogenization, to create a uniform solution and ensure the active ingredients are evenly distributed throughout the solution.
Detailed batch records are maintained to track the manufacturing process for quality assurance and regulatory compliance. In fact, ophthalmic products must meet regulatory requirements and quality standards established by health authorities, such as the Food and Drug Administration (FDA) in the United States or the European Medicines Agency (EMA) in Europe. Equipment must adhere to Good Manufacturing Practices (GMP) and undergo inspections for compliance.
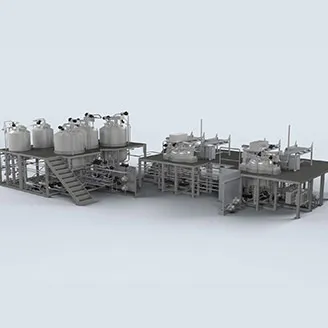
Olsa has extensive experience in the design and implementation of equipment and complex plants to manufacture ophthalmic eye drops, ranging from water-like to low-viscosity solutions, as well as ophthalmic ointment and gels.
Olsa provides equipment for ophthalmic products spanning from 30 liters up to 20,000 liters, always ensuring product sterility is maintained through the entire process, from initial equipment design to final execution.
Thanks to the synergy with MGA (Masco Group Automation), Olsa can provide an automation package to guarantee process control and related reporting.
Other Biopharma processing applications