Biopharma processing
Parenteral formulation
Overview
Overview
Parenteral formulation is a crucial aspect of pharmaceutical development, involving the preparation of sterile drug products administered through injections, infusions, or implants.
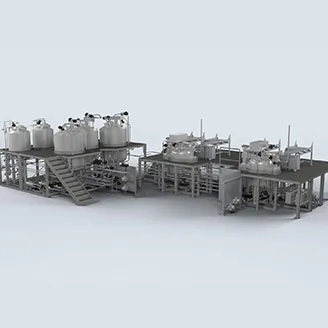
Large Volume Parenteral and Small Volume Parenteral (LVP and SVP) manufacturing involves producing sterile solutions.
The process starts with meticulous water purification and filtration to ensure sterility (Water for Injection). Active pharmaceutical ingredients (APIs), often dissolved in purified water or saline, are then carefully mixed and filtered to remove impurities. The solution undergoes sterilization, typically achieved either through terminal sterilization or aseptic processing, which ensure the solution remains sterile when transferred into large containers.
The choice of dosage form depends on the specific drug and its therapeutic purpose. Common parenteral dosage forms include solutions, suspensions, emulsions, and lyophilized powders.
In summary, parenteral formulation is a meticulous and highly regulated process that demands expertise in pharmaceutical science, microbiology, and quality assurance.
Over the past decade, Olsa has successfully delivered over 20 SVP/LVP plants, ranging in size from 200 liters to 200,000 liters.
Our innovative pre-assembled modular approach has significantly reduced both startup time and validation efforts in the project where this application was included.
Thanks to the synergy with MGA (Masco Group Automation), Olsa can provide an automation package to guarantee process control and related reporting.
Other Biopharma processing applications